With digitalization growing by leaps and bounds, the world of healthcare has witnessed a lot of changes over the last few years. From the emergence of smart medical devices and software apps to cloud-based data platforms and electronic health records — all of this has enabled better treatment and health monitoring both inside and outside clinics and hospitals. A new page has recently been turned by the innovative tech solutions that come under the heading of digital therapeutics (DTx).
This article will explain what digital therapeutics are, how these products are different from other digital health technologies, when to use them, and why you should care about the topic in the first place.
What are digital therapeutics?
Digital therapeutics (DTx) are high-quality software systems that help patients cope with various medical conditions and illnesses through health-related tips, behavior recommendations, exercise plans, meds intake alerts, etc. Not unlike drugs, DTx products provide clinically proven results that impact a condition. This is what separates DTx from a bunch of other wellness apps and medication reminders. There’s a wide array of disorders and diseases DTx products cover, including obesity, ADHD (attention deficit hyperactivity disorder), type 2 diabetes, anxiety, depression, congestive heart failure, and many more.
The Digital Therapeutics Alliance defines digital therapeutics as a new digital health category that "deliver[s] medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of diseases and disorders.”
So, digital therapeutics may function as
- a set of preventative care activities for patients at increased risk of chronic or severe diseases, e.g., weight control and exercise tips for people at risk of developing diabetes;
- sources of health information for making diagnosis and treatment decisions, e.g., daily reports submitted by patients suffering from depression;
- standalone treatments or those coupled with traditional therapies (in-person and/or pharmacological), e.g., digital programs for smoking cessation; and
- tools for monitoring and tracking symptoms aiming to continually improve treatment programs and health conditions, e.g., blood pressure control of patients with hypertension.
Many digital therapeutics make use of artificial intelligence (AI), machine learning (ML), and natural language processing (NLP) technologies to deal with patient data.
Digital therapeutics key principles
According to the above-mentioned DTx Alliance, in order to be called digital therapeutics, products must follow a set of principles, namely to:
- prevent, manage, or treat medical disorders or diseases, defined by ICD-10 codes for diagnoses;
- produce a software-driven medical intervention;
- use best practices for product design, creation, deployment, management, and maintenance;
- involve end-users in the processes of product development and testing;
- incorporate patient privacy and security protections;
- be validated by appropriate regulatory bodies;
- be published in peer-reviewed journals with trial results and clinically proven outcomes;
- make claims compliant with clinical evaluation and regulatory status; and
- collect, analyze, and apply real-world evidence as well product performance data.
Potential benefits of digital therapeutics
Though a relatively new concept, digital therapeutics promise tons of advantages for physicians, care providers, patients, distributors, and other stakeholders.
Patients receive personalized care and treatment programs, can access care from remote areas, and increase treatment effectiveness through better adherence to therapy.
Healthcare providers get opportunities to monitor patients in real-time, make timely interventions, improve the efficiency of care delivery, and reduce the need for personal visits by managing people with chronic conditions remotely.
Distributors are given a chance to keep track of the demand for different drugs regionally and nation-wide, check the usefulness of the drugs, and manage the supply chain more effectively.
Payers can reduce the costs related to care activities, increase sales, and improve patient experience and health outcomes.
Digital therapeutics bring innovations capable of filling in the gaps in the traditional medicine market and enhancing healthcare delivery in many areas. In the long run, DTx products have the potential to enhance the existing healthcare system significantly.
How digital therapeutics relate to digital health, digital medicine, and telehealth
While there’s an overlap between all these terms, they entail different therapeutic interventions and products. Besides, products coming under different categories have their own specific requirements for regulatory oversight and clinical evidence. It is necessary to make a distinction between digital health, digital medicine, and digital therapeutics to draw a clear picture of how different products can be utilized in the market.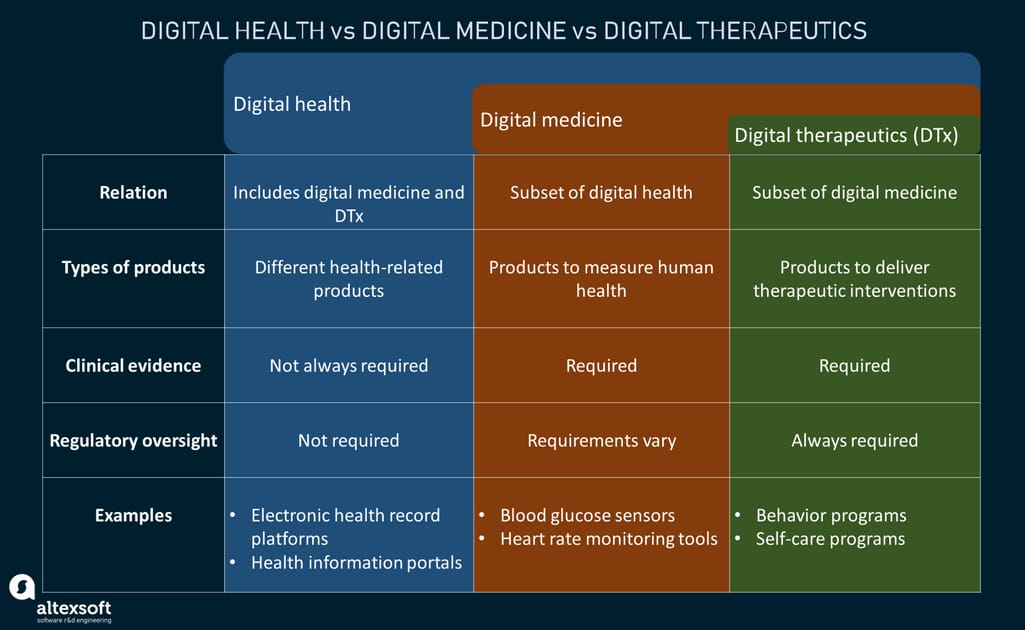
Digital health, digital medicine, and digital therapeutics compared
Digital health refers to the use of a plethora of health-related technologies, services, and products intended to enhance human health and make healthcare overall more proactive. It is an umbrella term for all concepts that bring information technology and healthcare together. As the broadest, multidisciplinary concept, digital health includes digital medicine, digital therapeutics, and telehealth. Digital health products that aren’t classified as medical devices don’t require regulatory oversight.
Digital medicine is a narrower field within digital health that involves the use of technology-driven products as tools for the measurement of human health and interventions in its service to support the practice of medicine broadly. In many cases, digital medicine deals with pharmaceuticals combining prescription medications and ingestible sensors. Consequently, such products often require certification by regulatory bodies, unlike digital health.
Digital therapeutics is another narrowly-focused digital health subset that, as stated above, employs various hardware and software systems to provide evidence-based therapeutic interventions for patients with different behavioral, mental, and physical conditions.
It’s also worth defining what telehealth is to avoid confusion. Telehealth is any means of providing long-distance, tech-enabled healthcare. It is considered the channel connecting health providers and patients. Telehealth can be a part of any of the above-mentioned scenarios, including digital therapeutics.
Now that you know the differences, let's look at the forms of digital therapeutics currently in existence.
DTx use cases
As stated above, DTx is an emerging category of digital health for preventing, managing, and treating diseases through changing patient behavior and remote health monitoring. Such products, for example, can be used to encourage patients to adhere to a certain exercise routine, diet, or drug regimens. And unlike common wellness tracking applications that often target various conditions, digital therapeutics mostly focus on one condition.
As a rule, patients make use of digital therapeutics through different software applications that can:
- provide some essential guidance, e.g., first aid techniques or instructions on how to cope with insomnia;
- augment conventional medication intakes assigned by physicians, e.g., asthma treatment;
- promote behavioral change through cognitive and motivational stimulation, e.g., cognitive behavioral therapy for patients with mental disorders;
- collect and analyze patient data so clinicians can personalize treatment regimens; and
- connect with different wearable and non-wearable medical devices to track and record vitals; e.g., tracking blood sugar levels.
Let’s take a closer look at some real-life examples of digital therapeutics.
FDA-approved digital therapeutic for substance use disorder treatment
Pear Therapeutics company developed two Prescription Digital Therapeutic (PDT) products that were among the first to receive regulatory approval from the FDA (Food and Drug Administration) to improve disease outcomes.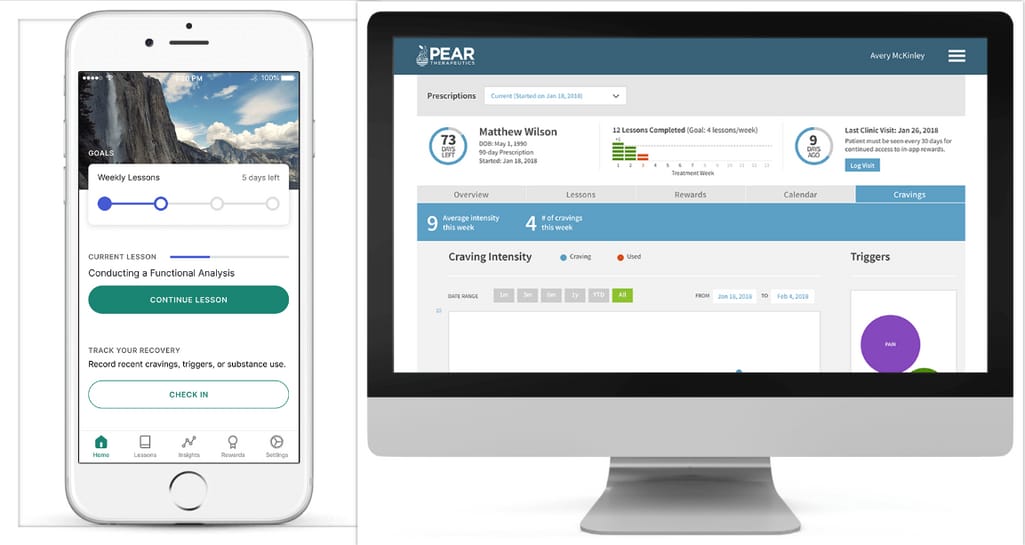
reSET application patient and clinician dashboards. Source: Pear Therapeutics
reSET is a 90-day PDT for people over the age of 18 having Substance Use Disorder (SUD). The product uses clinically proven Cognitive Behavioral Therapy (CBT) to enable patients with SUD to develop new skills, reach positive thinking, and avoid things that can lead to relapse. Patients get the app where they complete daily lessons, answer quiz questions, and report their substance use, cravings, and triggers.
reSET-O is a similar product, but it focuses on treating Opioid Use Disorder. Neither reSET or reSET-O are intended to be used as standalone therapy but as a complementing solution under the supervision of a clinician.
DTx program for diabetes management
Omada Health delivers digital behavioral interventions for diabetic. Doing so, they can reach more people than treatments done the traditional way – face to face. Omada’s digital health program leverages connected medical devices to track the activity, weight, and nutrition of patients to help them manage their glucose levels. It also incorporates training, personalized health coaching, and ongoing support of certified diabetes care specialists.
Digital therapy platform for heart failure medication monitoring
Biofourmis is a digital therapeutics company that augments personalized care and therapies to help patients with chronic conditions.
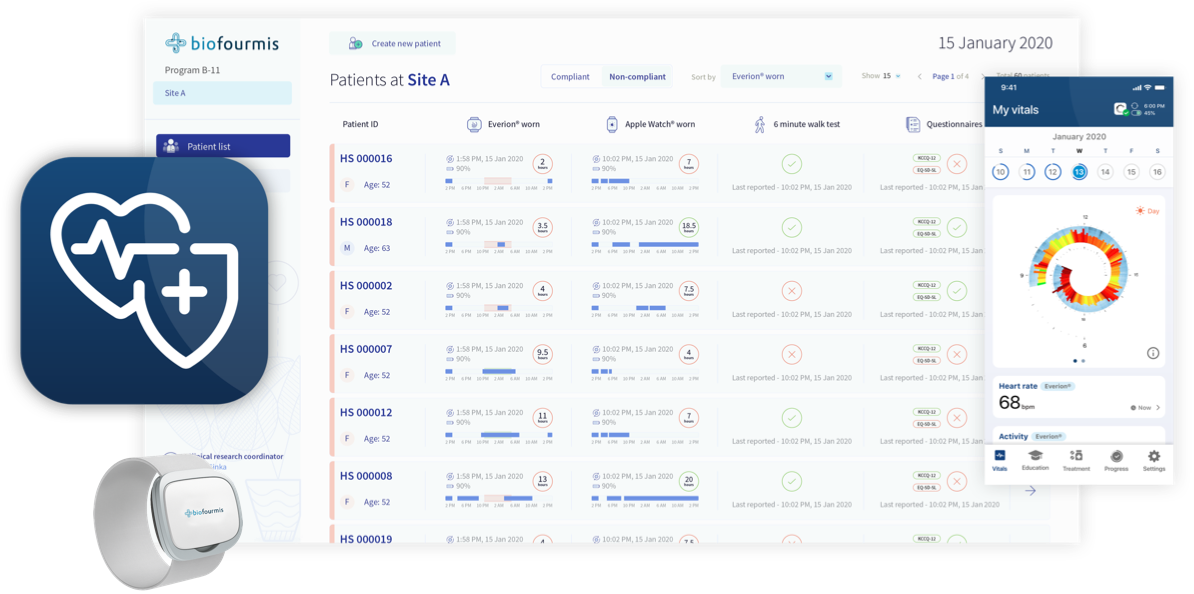 BiovitalsHF software screens. Source: Biofourmis
BiovitalsHF software screens. Source: Biofourmis
The company's main product is BiovitalsHF — prescription-available software that leverages AI and data analytics to detect early signs of heart failure by comparing physiological signals from a wearable sensor with data from other chronic patients. This allows doctors to make more accurate predictions and meds recommendations, which eventually helps manage patients with heart failure more effectively. Recently, the DTx product has received an FDA breakthrough device designation.
Prescription digital therapeutics to treat the symptoms of depression and anxiety
Happify Health, a software-enabled healthcare platform focused on improving mental health, launched their prescription digital therapeutics solution called Ensemble to help people diagnosed with Major Depressive Disorder (MDD) and/or Generalized Anxiety Disorder (GAD). Based on cognitive-behavioral therapy (CBT), the product should be prescribed by a clinician as a complement to conventional care for MDD and GAD.
With Ensemble, patients get the opportunity to deal with MDD and GAD by learning new skills, turning them into habits, and changing negative thinking patterns. Patients communicate with an AI-powered digital mental health coach named Anna by answering her questions and following her guidance. The app also provides patients with a set of daily activities sequenced in a way that will encourage the desired outcomes.
VR technology for psychosis treatment
The VR system called gameChange aims at transforming and improving the lives of people who struggle with psychosis by enabling state-of-the-art virtual reality therapy.
A shot from the gameChange VR system imitating the situation in a doctor’s waiting room. Source: gameChange
For individuals with psychosis, even such common social situations as visiting a doctor or doing shopping may provoke anxiety and stress. Thinking bad things are going to happen to them, people often withdraw socially. The gameChange VR system simulates different social scenarios patients fear to help them learn new behavior patterns and help them feel safe.
How to approach digital therapeutics
Companies that want to unlock the new chapter of medical innovation called digital therapeutics should take into account all the steps from product research and strategy outline to design and development.
Research: deciding on the DTx solution type
According to Deloitte, companies can investigate two potential areas to decide on the DTx R&D: One is to go after a specific digital use case and the other is to pursue a specific therapeutic area.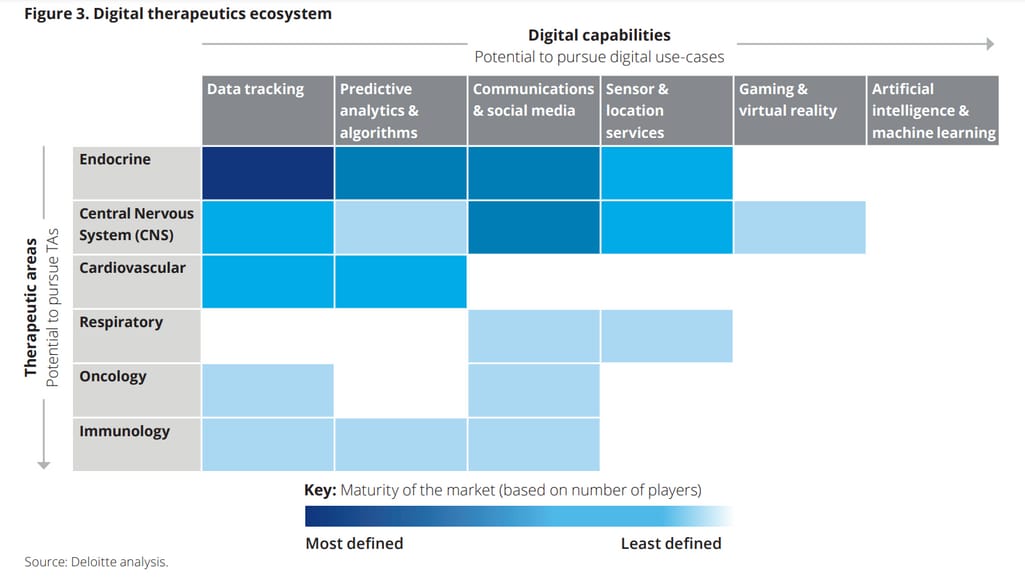
Opportunities the digital therapeutics landscape offers. Source: Deloitte
Scenario focused on the digital use case. This scenario revolves around the idea of how a certain digital capability can be used as a treatment. For example, a decision can be made in favor of building a DTx solution that leverages VR technology to recreate environments and stimulate certain types of behaviors within them. While the scenario is promising and transformational, it can be more difficult to accomplish as it requires a more dedicated approach to solution development and greater investment.
Scenario focused on the therapeutic area. Within this scenario, MedTech and pharmaceutical companies decide on what DTx product to build based on their clinical and therapeutic expertise. They choose a specific therapeutic area that requires new approaches to complement or replace existing treatments.
Strategy: deciding on how to build the product
After deciding on the type of DTx product, MedTech and pharmaceutical companies have to choose how they are going to build the project.
Developing DTx software may involve in-house and in-licensing scenarios.
In-house development. Large companies that have enough financial resources can go for in-house development. In the first place, they need to find and hire professionals including
- clinicians specializing in a disease the app is meant to treat,
- experts in clinical trial design,
- software engineers,
- data scientist (if you are planning to add AI capabilities),
- product managers,
- regulation experts, and more.
The process requires a great deal of cross-functional collaboration during the whole cycle of product design. Click Therapeutics has chosen a path of developing products on their own platform.
In-licensing development. This approach appeals to DTx new-comers or companies that want to expand their DTx portfolio without hiring additional workforce. Instead of starting the project from the ground up, they can invest in tech development or ongoing research performed by a third party.
For example, digital treatment startup Mahana Therapeutics signed a contract with King’s College London to in-license a three-month self-management program for patients with irritable bowel syndrome (IBS.) In all, the therapy development took 18 years of research and clinical testing. For Mahana Therapeutics, the agreement was a great opportunity to receive clearance and launch their first evidence-based DTx product within a year.
This shortcut is also beneficial for academic labs and research centers that often lack money or skills to complete their study and bring it to market in the form of patient-centric software.
Software development: turning academic assets into DTx solutions
No matter the business strategy, DTx product development includes the following phases.
Everything starts with the pre-clinical phase involving the development of a therapeutic intervention that will further be converted into a digital format. This can be a therapeutic intervention that exists in the scientific literature or already is undergoing clinical trials.
Then comes the trial version of the DTx software producing data that will make it possible to evaluate the therapy impact and the opportunity of its further development. The first test will assess the usability and accessibility of the solution by patients. Then the pilot clinical study will take place on a narrow group of patients. The conditions of use will be controlled by specialists.
Based on the results of the pilot stage, the product will either enter the full development stage, including technical and commercial aspects, or will be shut down. If the pilot’s a success, the solution will also go through more controlled clinical trials to prove its value for regulatory authorities, physicians, and insurance companies.
Major challenges that digital therapeutics face
Implementing and commercializing DTx solutions involves a set of complex tasks that concern not only numerous decision-making processes but also the participation of stakeholders from different fields. Companies need to be aware of four major challenges that often accompany the adoption of the digital health ecosystem in general and digital therapeutics in particular.
Regulatory approval. A major DTx hurdle is how intensely government bodies such as the US Food and Drug Administration (FDA) scrutinize them. Digital therapeutics are quite diverse. Accordingly, there is no universal framework for control structures to evaluate and approve these products. So far, digital therapeutic products fall under two larger categories.
- Software as a Medical Device (SaMD) is software covering one or more medical purposes and performing those purposes without being a part of a hardware medical device.
- A Mobile Medical Application (MMA) is a mobile-tailored software that is an extension of or accessory to medical devices or one that turns a smartphone/tablet into a medical device for diagnosing, treating, or monitoring diseases.
To modernize the regulatory oversight of software-based medical devices, the FDA launched a new regulatory pathway — the Software Precertification (Pre-Cert) Pilot Program — in 2017. Currently, nine companies out of 100 applicants participate in the program.
Clinical validation. Another challenging part in regard to implementing DTx is the need to go through clinical evaluation, meaning products provide a valid, reliable clinical outcome for a target clinical condition. Treatment effectiveness can be verified by comparing clinical results to no treatment, to a minimum standard of care, or the “gold standard” of treatment.
Physician and care provider adoption. To encourage mass adoption of the digital therapeutics, physicians and care providers must be introduced to them. They need to be aware of the available solutions and their outcomes they can make available to their patients. To do this, some DTx companies use the pharmaceutical model, in which distribution partnerships are leveraged to inform physicians of solutions.
Unawareness of DTx opportunities and options. The digital therapeutics sphere is not a mature one and so not well known to the market. Even with ever growing interest, many consumers are unaware of what types of DTx treatment exist. This is one more roadblock on the way to embracing DTx solutions on a larger scale.
Data privacy. Digital therapeutics are driven by large amounts of highly personalized patient data. Providers need to build solid frameworks to ensure data privacy and security so that DTx products can reach a wider market.
What’s on the horizon for digital therapeutics?
When it comes to innovations, it’s not only the present that matters but also the future. In the interview with McKinsey, Bozidar Jovicevic, head of digital therapeutics at Sanofi, expressed the following opinion, “And ten years from now, I’d love to see prescribing and using digital therapeutics become just a standard of care — a normal thing, like prescribing a drug. That would reshape the practice of medicine.”
The truth is, this belief has a great chance of becoming reality. Digital therapeutics have already proved to be effective through driving behavioral and lifestyle changes of patients having chronic illnesses and conditions, including asthma, ADHD, diabetes, etc. Studies like the one presented by the American Journal of Preventive Medicine confirm that approximately 30 to 50 percent of a person’s health is driven by lifestyle and habits. With digital therapeutics, it will be possible to treat patients in a completely different, new way – by gradually changing how people take care of their health.
Also, digital therapeutics have a huge potential in areas where demand for treatment outgrows its capacity. For example, if you look at the US, you will see that the country’s population is aging and driving greater demand for medical care. People aged 65 and older, who make up 16.5 percent of the US population, now account for 34 percent of the demand for physicians. According to the AAMC (Association of American Medical Colleges), this demand will account for 42 percent by 2034, outpacing supply and resulting in a shortage of physicians. Early diagnosis of diseases along with timely digital therapeutic interventions have what it takes to solve this problem.
It’s fair to claim that as more digital therapeutics solutions are created, there will be more therapy options addressing a greater number of behavioral, mental, and physical issues in the future.

