When it comes to medicine, on-time delivery can literally save someone’s life. Not only do medical products have to be transported on schedule, they must also be stored and handled correctly at every stage of the journey. To make sure hospitals and clinics have what they need to treat patients, medical supply chains must be closely managed and optimized for utmost performance.
This article talks about the unique challenges of healthcare logistics management and explains how technology can make it more efficient. So whether you are a medical/pharma company looking for ways to enhance your supply chain or a logistics business expanding your range of services, we hope you’ll get valuable insights from reading.
What is healthcare logistics?
Healthcare logistics involves managing the flow of medical supplies, pharmaceuticals, and medical devices from manufacturers to healthcare facilities and, ultimately, to patients. It also relates to handling other healthcare-related items such as lab specimens, organs, veterinary medicinal products, etc.
Logistics is a critical part of the healthcare system because it ensures that the right products arrive in the proper condition, at the right time, and in the right place. This is vital for patient care, as delays or errors can lead to disrupted treatment, increased healthcare costs, or even harm to patients.
For you as a company that deals with medical shipments, mishandling can also have serious business outcomes, impacting your bottom line, brand image, and market share. So setting up the right supply chain workflows is crucial – and that’s where technology comes to the rescue.
Before we discuss which tech advancements can support and enhance your operations, let’s define the main complexities of medical supply chains.
Healthcare logistics needs and challenges
As medical shipments travel from the manufacturing plant or other place of origin to their destination, they pass through a variety of environments and handlers. The healthcare industry is unique in terms of the numerous regulations that define strict handling guidelines for every stage of their lifetime. Many medical products are temperature-dependent, some are hazardous, and some have a very short shelf-life.
Because of their variety, different healthcare items heve multiple journey scenarios. For example, blood samples or other specimens obtained in a clinic must be packaged right there and transported to the nearest lab with no storage needed. On the other hand, say, pharmaceuticals are manufactured in a factory, packaged, transported to a warehouse for storage, and then shipped to a hospital, pharmacy, or patient.
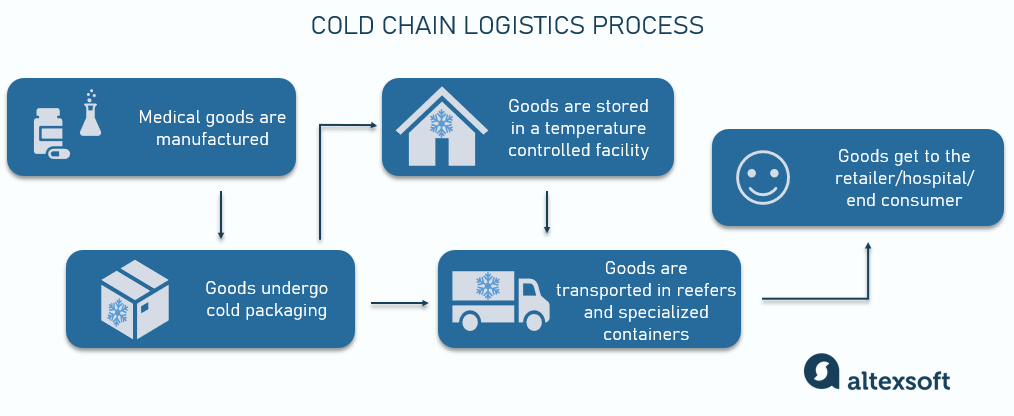
However, whatever their journey stages are, there are some common pain points relevant to almost any type of medical product, i.e.,
- control of temperature and other conditions,
- respecting the expiration dates,
- tracking shipments and monitoring their condition, and
- regulatory compliance.
Please note that in our overview, we’ll focus on the industry-specific challenges, but it’s important to be mindful about other complexities typical for any supply chain, such as demand planning, space optimization, or supplier management.
You can refer to the Transportation section of our blog to gain insights into tech-driven warehouse management, inventory management, transportation management, and other logistics aspects.
Control of temperature and other conditions
As per MarketResearch.com, over 95 percent of all approved biologics and 90 percent of all vaccines are temperature-sensitive. It means that it’s critical to maintain strict temperature requirements when storing, handling, and transporting such medical products.
There are several standard temperature ranges:
- controlled room temperature – 15° to 25°C (59° to 77°F),
- cool storage – 8° to 15°C (46.4° to 59°F),
- cold storage – 2° to 8°C (35.6° to 46.4°F),
- fridge storage – -4˚C to 2˚C (24.8° to 35.6°F),
- frozen storage – -15˚C to -20˚C (5° to -4°F),
- ultra-frozen storage – -70˚C to -80˚C (-94° to -112°F), and
- cryo storage – -160˚C (-256°F).
In addition to temperature limitations, humidity, ventilation, light, vibration, and other requirements often exist. Not to mention sterility, of course.
Plus you might also be dealing with highly active, radioactive, and hazardous materials that present special risks and require additional safety and security measures.
All these requirements are extremely difficult to comply with. Nearly half of Biopharma ColdChain Logistics Survey respondents reported multiple temperature excursions annually. The same research also mentions that IQVIA Institute for Human Data Science estimated loss in the pharma industry to be about $35 billion annually due to improper handling of temperature-sensitive medical shipments.
So one of the biggest challenges of healthcare logistics is maintaining the proper conditions throughout the entire shipment journey. Here’s what happens at different stages.
Storage. Depending on which type of medical products you deal with, your warehouse facility must have robust climate control to comply with standards. In most cases, there must be certified storage and loading areas that maintain the required conditions, such as walk-in cold rooms (WICR) or ultra-low temperature (ULT) freezers.
Also, many storage facilities implement a dry ice production system to supply this common cooling agent constantly.
Packaging. To transport temperature-sensitive medical products safely, they must be put in special containers. There are two main options.
Passive containers are basically insulated boxes that maintain the internal temperature with the help of special coolants (e.g., dry ice, gel packs, gel bottles, liquid nitrogen, etc.). Active containers, however, have electrically controlled cooling systems. They monitor the internal temperature with a connected sensor and automatically adjust the conditions to maintain a stable environment.
Transporting. In addition to proper packaging, sometimes, medical shipments demand specialized equipment known as reefers or freezers. Depending on the mode of transportation, it can be refrigerated trucks, railcars, cargo ships, and aircraft. If not only temperature but also humidity must be controlled, such equipment has to be capable of that as well.
Please read more about cold chain logistics and handling temperature-sensitive shipments in our dedicated article.
Respecting the expiration dates
Expired medical products are, at best, less effective, and at worst, present a significant danger for the patients. For pharma and medical companies, expired medicine is also a source of substantial financial losses. For example, just one drug – Pfizer’s antiviral medication Paxlovid – cost the EU over a billion dollars because millions of doses were left unused by their expiration date.
When you deal with large volumes of various medical supplies, expiration management becomes a complex issue. To minimize losses, inventory management suggests implementing the FEFO (first expired, first out) and/or FIFO (first in, first out) approaches. There are also various practices such as cycle counting and inventory rotation that help monitor expiration dates. However, when performed manually, all these techniques aren’t perfectly reliable as they are too prone to human error.
Tracking shipments and monitoring their condition
Closely related to the previous two, there’s a big need for real-time, accurate tracking of medical shipments' location and condition. In 2024, calling drivers and asking them to check the temperature inside the container and report where they are just doesn’t work. You have to obtain precise information at any moment in time to be able to react in case of disruptions.
Regulatory compliance
Regulatory compliance in healthcare logistics is extremely complex because of the critical nature of medical products, the global landscape of supply chains, and the diverse regulatory environments across different jurisdictions.
Here are the most important global regulations that relate to medical supply chains.
Good Distribution Practices (GDP) ensure the quality of medicinal products is maintained throughout the supply chain, from the point of production to the point of sale or distribution to the end user.
Good Storage Practices (GSP) are a part of GDP, focusing on the proper storage conditions and management of pharmaceutical products.
Good Manufacturing Practices (GMP) are about compliance with quality standards in all aspects of production – from the starting materials, premises, and equipment to the training and personal hygiene of staff.
EU Falsified Medicines Directive (FMD) aims to protect the pharmaceutical supply chain from counterfeit medicines in the EU. It includes measures such as safety features and a unique identifier for prescription drugs.
ISO 9001 and ISO 13485 Standards provide guidelines for the quality management of medical products and devices. You might need to obtain the corresponding certificates together with Drug Distributor Accreditation (formerly VAWD).
See Also
There are also WHO’s Guidelines on the international packaging and shipping of vaccines, IATA’s Perishable Cargo Regulations and Temperature Control Regulations, the FDA’s Hazard Analysis Critical Control Point (HACCP), and many other provisions. All of them ensure that healthcare logistics operations are conducted in a manner that preserves the integrity and safety of medical products – which is great, but complying with them is a constant pain in the neck.
Now that we’ve highlighted the main hardships of handling medical shipments, let’s see how technology can help you deal with them.
How modern technologies enhance healthcare logistics
Today, a number of technologies and software systems can be implemented to enhance logistics processes and build a more resilient supply chain.
IoT for proactive temperature monitoring
As we said, temperature control is perhaps the most critical challenge for healthcare logistics – especially during last-mile delivery when temperature fluctuations happen most often.
The use of specialized storage, packaging, and transportation equipment is essential, but digital technologies can also be of great value. They help continuously monitor temperature, humidity, and sometimes even vibration, giving stakeholders information that a safe and stable environment is maintained.
Data loggers are devices placed inside the container or storage area that record temperature data at specified intervals. They have to be scanned to retrieve a detailed storage and transit conditions history for compliance and quality assurance.
Though most cost-effective, data loggers aren’t capable of real-time monitoring, so they are mainly used for non-critical shipments such as standard pharmaceutical products.
IoT infrastructure based on BLE (Bluetooth Low-Energy) or other types of sensors is a more advanced solution. A system of connected devices attached to containers or packages collects temperature measurements and location data in near real-time and wirelessly transmits all the records to the cloud storage. There, information is processed and can either be retrieved when needed or sent as live updates to a user-facing app or platform.
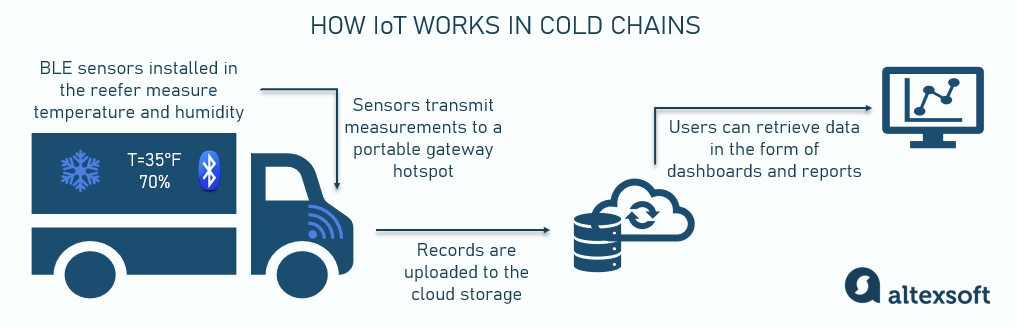
The value of real-time monitoring lies in automatic alerts in case of any deviations from predefined temperature ranges (or other conditions), allowing for immediate corrective actions.
Going a step further, you can also employ machine learning (ML) to analyze collected data and find deviation patterns, for example, if temperature breaches happen regularly at a specific stage. It can help you define bottlenecks or process inefficiencies, improve operations – and thus minimize costly incidents.
Predictive maintenance for equipment support
One more way to prevent temperature breaches is to ensure all storage equipment like coolers, freezers, or ice machines functions properly at all times. But just like any other piece of machinery, it’s evident that equipment requires consistent maintenance or repair in case of failures.
To foresee the need for repair and minimize downtime, employ a predictive maintenance approach. Just like temperature control, it requires developing an IoT infrastructure with connected sensors continuously monitoring equipment health, analyzing collected data, and alerting maintenance staff of required actions.


Inventory software for expiry management
To manage the expiry of medical products, it’s worth implementing the practical methods we mentioned (inventory rotation, FEFO approach, etc.) and supporting them with inventory management software. This way, you’ll automate part of the workflows, improving their speed and accuracy.
For example, using asset tracking solutions like barcode, RFID, or BLE tags will allow you to capture essential product information, such as production date, expiration date, lot number, location, and status. Having all this data in one system, you’ll be able to track product expiry and see which items must be moved first. Moreover, you can set up alerts that will automatically notify you about upcoming expirations so that you can act accordingly.
Inventory management software also helps analyze inventory levels and optimize replenishment by applying advanced, ML-based demand forecasting techniques. If you are a distributor, optimal stock levels will allow you to efficiently use storage space and ensure medical product freshness.


Telematics and GPS for real-time tracking
For peace of mind regarding the location of your time-critical medical shipments, leverage GPS tracking technology. If you truck healthcare products, you can get real-time location data from telematics devices installed in vehicles.
If you ship by air or sea, you can get tracking data from the carrier. In most cases, it’s provided on their website, but if you want more detailed, real-time information, you can integrate with their system and get status updates automatically.
Quality management software to ensure compliance
Regulations provide guidelines regarding all logistics aspects, e.g., warehousing and handling practices, temperature monitoring activities, disposal process, human resources, reporting, etc. In addition, these regulations sometimes change, which makes compliance even more complicated. To ensure consistent conformity with all the essential healthcare provisions, you can implement quality management software or QMS.
QMS is a digital platform that helps healthcare organizations manage and improve the quality of their services and operations. Its functionality supports quality assurance, compliance, risk management, performance improvement, and other essential operational aspects. Here are some of its features related to logistics.
Documentation management. QMS helps keep quality standards, policies, and procedures up to date and accessible to authorized personnel.
Compliance management. Tracking changes in regulations, the QMS helps ensure that logistics and other practices are aligned with current standards and requirements.
Risk management. QMS provides tools for identifying, assessing, and managing risks throughout the organization. This helps in prioritizing risks based on their potential impact on patient safety and organizational performance.
Supplier management. QMS helps manage supplier qualifications, performance evaluations, and quality audits.
Audit management. QMS supports the planning, execution, and documentation of internal and external audits. This ensures that the organization regularly reviews and improves its quality and compliance processes.
There are definitely many other types of digital tools that can enhance different aspects of your supply chain. For example, automated sorting systems speed up and increase the accuracy of picking and packing processes. Meanwhile, access control systems help prevent the handling of important shipments by unauthorized staff and give you an understanding of personnel activities.
To choose the right set of tools, you must first define your key pain points and inefficiencies. Then, as you implement software, configure it to address your specific needs and cover the problematic workflows.
For maximum efficiency, integrate all your IT components (data sources, digital tools, processing units, etc.) into one connected structure. It will allow you to establish seamless information flow throughout your organization, ensure end-to-end visibility, and enable data analytics.
Digitizing healthcare supply chains: success stories of Pfizer, Eli Lilly, and Novartis
Many logistics service providers today offer comprehensive supply chain management to medical organizations, so a lot of them leverage the professional expertise of 3PLs. The recent UPS Pharmaceutical Industry Study states that 42 percent of manufacturers in the healthcare industry prefer to outsource temperature control, while 34 percent are willing to handle it on their own.
However, even in spite of outsourcing logistics operations, big pharma companies tend to digitize their supply chain-related operations as part of the overall transformation. Let’s look at some of the examples.
Pfizer has introduced the Pfizer Global Supply – Digital Operations Center (DOC) to support its manufacturing and entire value chain as COVID-19 struck. The DOC leverages the most innovative technologies, including AI, augmented reality, and IoT. As a result, the pharma giant has an end-to-end view of manufacturing and can predict and address issues before they appear.
In addition, Pfizer partnered with Controlant, a specialized pharma tech provider, to set up the IoT infrastructure for real-time shipment tracking and condition monitoring along the entire supply chain. All these efforts enabled the delivery of billions of vaccines worldwide with a close to 100 percent success rate.
Eli Lilly has been investing in developing big data infrastructure for years now to enable predictive analytics in all business aspects. They are also continuously working on their Digital Plant project to build the most advanced, technology-based manufacturing facility.
The company uses robots in its plants and warehouses and relies on IoT for condition monitoring and predictive maintenance. It also implements blockchain and digital twin technologies to improve supply chain traceability, logistics and inventory management, and quality and regulatory compliance.
Novartis, another leading pharma and biotech company, has been actively cooperating with the biggest global tech providers, such as Microsoft and Amazon, to build an enterprise-wide data platform, enable advanced data analytics, and make information accessible to every associate. Novartis applies AI/ML techniques to make accurate demand predictions and increase preparedness for any market fluctuations.
Novartis has also been working continuously on digitizing and automating its supply chains. For example, it partnered with warehousing solutions expert Mecalux to build a fully-automated storage and distribution facility in Poland. Such a holistic automation approach resulted in increased security, reduced maintenance costs, control of all movements, expanded storage capacity, and lower personnel costs.
It was also one of the companies that implemented Kinaxis’ RapidResponse platform to establish end-to-end visibility, support the planning processes, and enhance overall supply chain resilience.

Maria is a curious researcher, passionate about discovering how technologies change the world. She started her career in logistics but has dedicated the last five years to exploring travel tech, large travel businesses, and product management best practices.
Want to write an article for our blog? Read our requirements and guidelines to become a contributor.

