Precision medicine is a very new term that exists without the hype. You don’t see it screaming at you from headlines, startups are not using it on their landing pages, and it’s slowly climbing up Garner’s Hype Cycle for Healthcare Providers, not yet looming as large as IoT, blockchain, and analytics as they relate to healthcare. But it exists on the pages of peer-reviewed medical journals and research papers.
In 2015, a precision medicine effort was launched by Obama’s administration in form of the Precision Medicine Initiative - a research movement in the US aimed at changing the approach to disease prevention and treatment. Several years into the program, some initial successes are visuible with a rough implementation plan for providers ready to tap into the potential. This is what we will talk about.
What is precision medicine? Precision medicine vs personalized medicine
So how do we treat patients today? The modern healthcare system favors the average Joe. Each person is treated the same following the standard therapy. It doesn’t always work of course, and patients have to go through a few trial-and-error iterations before they get a treatment that truly helps and causes minimum adverse effects and harm. And getting that treatment is somtimes possible only if the person’s financial situation allows it - waiting for the right care is still a luxury for many.
Precision medicine (PM) targets not the diagnosis, but each patient, with their complex genome and history. Formerly called personalized medicine, the term was changed to avoid the misinterpretation that treatments would be developed specifically for each person. Precision medicine is aimed at finding the perfect treatment based on data. Data about a patient’s family health history, genetic characteristics, and even lifestyle. Similar to how Netflix, Facebook, and Amazon give us content and shopping recommendations based on what they know about us and their customers in general, healthcare providers can recommend procedures, drugs and their doses, and predict diseases using personal medical data.
Here’s how precision medicine will work:
- Different types of data about a patient is collected. This can be data from surveys, tests, and wearables.
- This data is standardized for different systems to understand.
- Data is shipped to the database or other type of storage, where it’s transformed for future use.
- Advanced analytics and algorithms use this data and data aggregated from other anonymous patients to come up with a diagnosis and care recommendations.
- Treatment results are recorded to improve algorithms.
- Initial data and treatment results are shared with research centers to help in developing innovations.
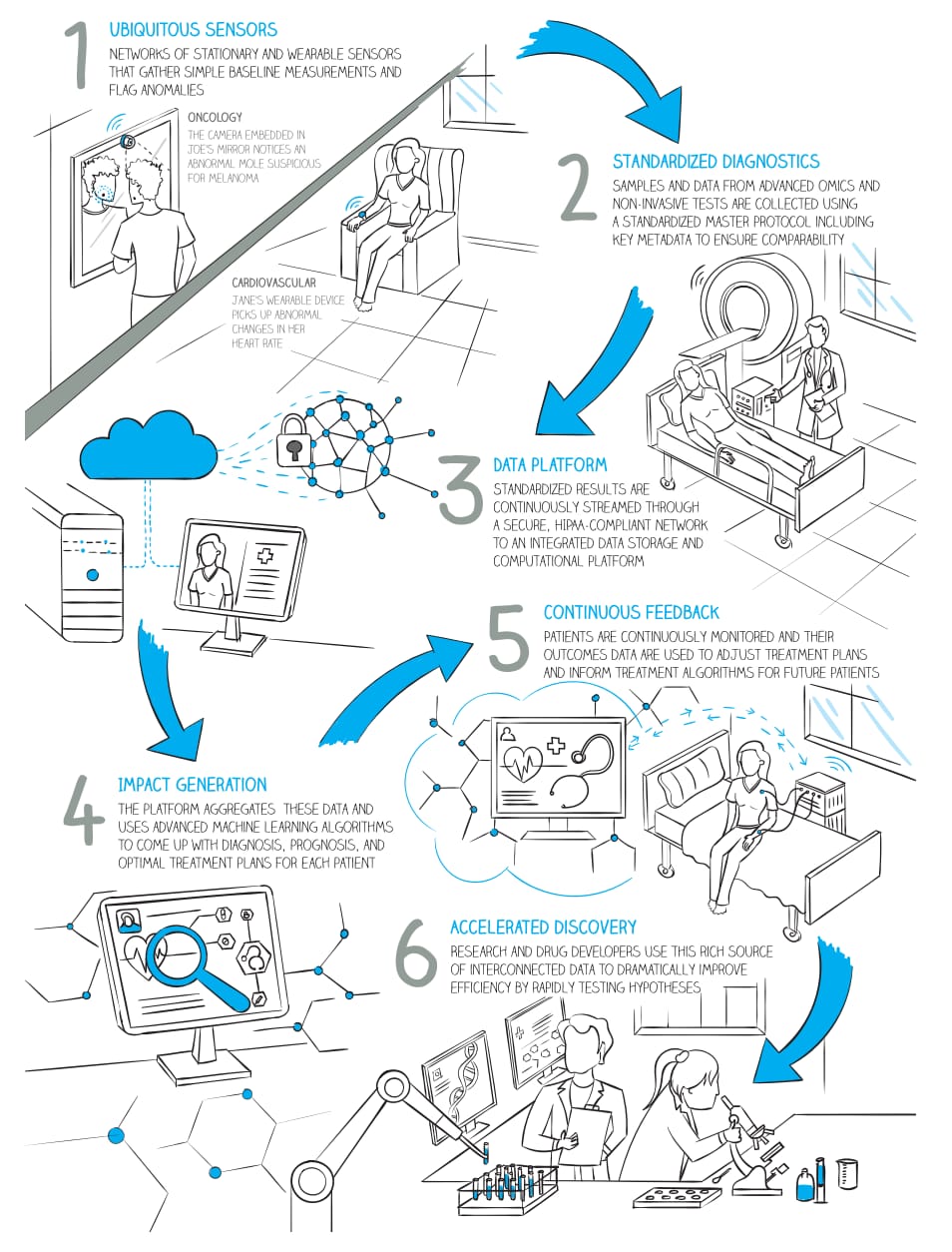
How precision medicine will work Source: McKinsey’s Precision Medicine Opening the aperture
Here’s an example. It’s known that 25 percent of pancreatic cancers have actionable molecular alterations that may benefit from a specific therapy. So, the Know Your Tumor program enrolled 1856 patients with pancreatic cancer to get molecular testing of their tumors and find these alterations. Patients received detailed reports with possible treatment options, approved drugs, and even experimental therapies available through clinical trials. One of every four patients was found to have molecular alterations and received personalized therapy. This allowed them to live on average one year longer compared to patients who didn’t get the matched treatment.
Total precision medicine is not yet available to everyone and currently exists in private research groups, generating evidence of its benefits. For it to work on a nationwide scale, we need a collaborative effort of all industry players. This is the effort we’re talking about.
Requirements for the adoption of precision medicine
True precision medicine will work through collaboration and free data sharing. This should be an ecosystem of patients, healthcare providers, pharma, research consortiums, labs, medical devices, and all health IT.
Here’s what’s been done over the last five years:
Progression in data availability. The industry succeeded in producing an unprecedented volume of medical data - it’s estimated that its volume is annually growing by almost 50 percent and it will reach 2,314 exabytes (or billion gigabytes) in 2020.
Growth in EHR adoption. More than 96 percent of hospitals in the US use an EHR system, creating a foundation for future technology adoption.
Evidence of PM success. Numerous studies and research projects disclosed the benefits of targeted therapies. There have been advances in developing machine learning algorithms for diagnostics.
McKinsey predicts that at least five more years is needed to complete the next round of developments. We covered the three biggest ones below.
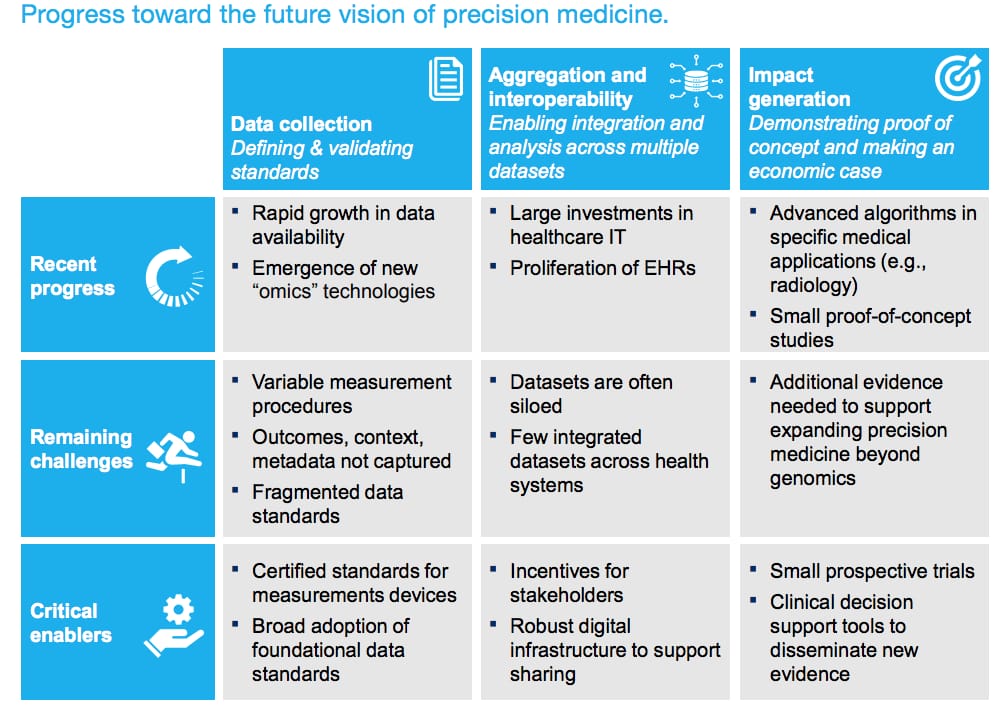 Standards, infrastructure, and decision making tools are the critical enables towards PM adoption
Standards, infrastructure, and decision making tools are the critical enables towards PM adoption
Transparent data collection and sharing
The reason why other industries have already been successfully utilizing Big Data is that this data is given to them willingly, by clicking a checkmark on the website. Surveys show that retail customers don’t mind sharing data in exchange for benefits like personalization. In healthcare, the rules are stricter. The new GDPR, CCPA, and HIPAA regulations have given people control over their data, while simultaneously stalling healthcare innovations. Data is siloed in hospitals, it often has to be destroyed after the study ends, and it’s nearly impossible to use when it’s been collected and stored in dozens of different formats.
In November 2020, the final interoperability rules will come into effect. These are nationwide standards by which healthcare providers and IT developers will be able to share data, do it safely, and without taking control from patients. These rules describe two standards: USCDI, which explains how data should be recorded, and FHIR, which explains how data should be exchanged. This is the closest we can get to uniformity in the industry and it’s an important step to enable technology to communicate freely.
Data analytics infrastructure
Once collected, raw data will find its way to analytical software, where algorithms will find hidden patterns, make calculations, and present findings in a user-friendly interface. We covered a few thought-provoking cases in our healthcare data analytics article. Analytical modules are common for EHRs, but they are rarely used to their full potential since most of them have to be customized, often by building a full-fledged data pipeline. In precision medicine, analytics will have to be even more sophisticated, using AI and real-time analysis.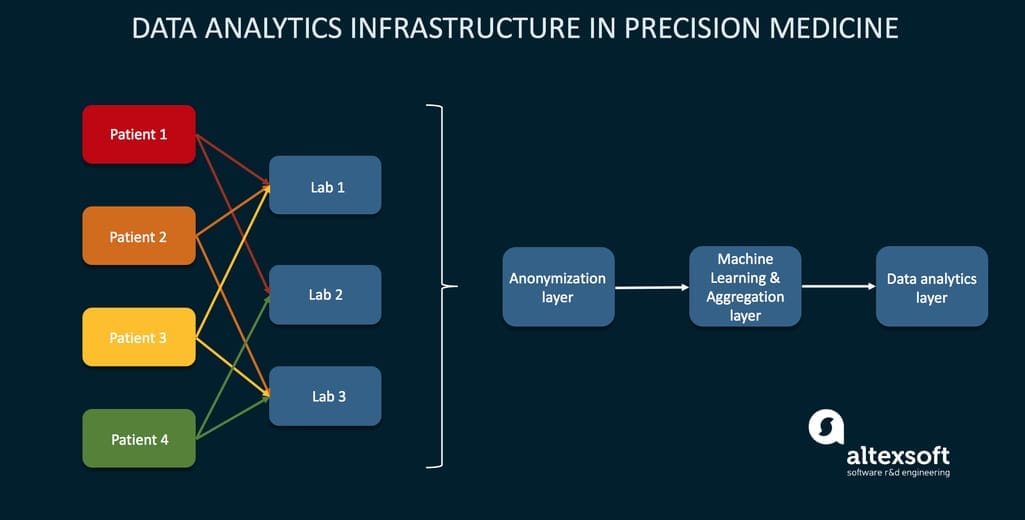
The exemplary infrastructure of analytics in PM using three layers Source: An infrastructure for precision medicine through analysis of big data
Analytics of such scale hasn’t traditionally existed in hospitals or even most research centers. But as our demand for it grows, industry players will start viewing it as a competitive advantage. Pharma companies, labs, and healthcare providers will either employ these solutions or contract a tech-adept third party to do analytics for them.
Decision-making technology
The third layer of the precision medicine process is data-driven decision making. This is similar to currently existing clinical decision support systems or CDSSs that help providers make treatment decisions based on the aforementioned analytics. But in precision medicine, it gets more effective and goes beyond patient care. For example, such technology would be also used for clinical trials to make better decisions on early study phases, save time and money, and lead to breakthroughs faster.
In hospitals, providers would be able to make more informed treatment decisions just because they get to compare patient data against huge databases of genomic data that weren’t available before. The plan is to build a network of hospitals, labs, and research consortia to have this data open and analyzed by algorithms whenever a physician needs to prescribe a new drug or make a diagnosis.
How hospitals can prepare for precision medicine adoption
In our article about EHR problems, we addressed issues that majorly hinder the successful use of EHRs in hospitals from poor usability or lack of automation. Despite its many issues, EHRs are actually our best source of data and the best shot at building a robust data exchange infrastructure. But since EHRs weren’t initially designed with research purposes in mind, the following changes must be made.
Step 1. Improve data recording capabilities
Data recorded in EHRs is usually not complete or detailed enough to use in a cohort-based study. Many EHRs are legacy behemoths that don’t have modern recording capabilities - information is often typed in narrative text rather than entered using classification codes. Besides, for precision medicine purposes, much additional patient information is needed, like a person’s diet, lifestyle, family health history, race, allergies, and maybe even data collected from wearable technologies. Before your EHR can support robust and coded data recording, this data can be rendered useless.
Step 2. Standardize patient data
Most EHR systems support the Common Clinical Data Set (CCDS) - a list of patient reporting standards required for getting a certification on health technology. It features such industry-known standards as HL7, SNOMED, LOINC, National Drug Code, RxNorm, and more. The final interoperability rules broadened them with two additional classes: clinical notes and extra information about the data creation. It’s time to actually start using these standards as they are to become industry-wide and will be a basis for precision medicine data.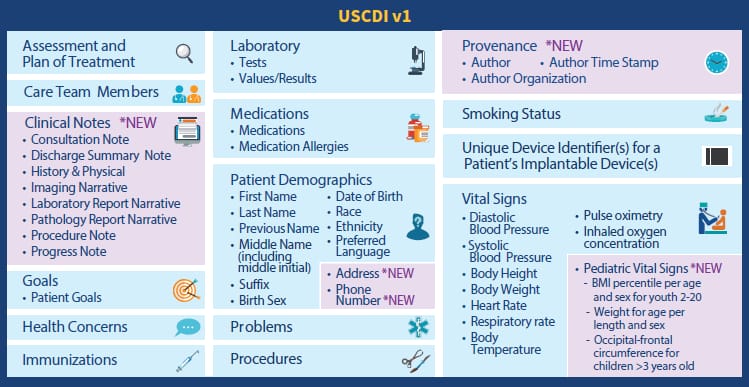
USCDI data classes and elements Source: HIMSS Report
Step 3. Integrate data access and sharing via APIs
The basic standard for sharing hospital data today is FHIR, which uses RESTful APIs in XML, JSON, and RDF to connect to different parties of the healthcare data exchange process. You can deploy FHIR services in the cloud using ready-made solutions like the one from Azure or build it on top of your EHR with the help of your IT contractor.
Step 4. Give genetics training to clinicians
As of now, genomic information is not widely used in hospital care. Genetic testing is available through technology companies like 23andMe, but in traditional care, there’s outdated thinking that genomics is mostly excessive and shouldn’t be included in routine testing and patient information. For precision medicine to succeed, this has to be changed. Genomic data should be collected along with such regular procedures as checking temperature, blood pressure, or a person’s height and weight. In the future, we expect to use EHRs for genomic research and there’s where such programs as eMERGE and ClinGen are directing their efforts. But what can hospitals do to prepare today?
You can start by overcoming barriers from clinicians. Primary care practitioners lack the confidence and ability to perform genetic tasks, counsel patients, and interpret test results. They also have a perception that “genetics is for specialists.”
You can start by bringing awareness of the benefits of introducing genetics into primary care. Then, they need to be assured that no long-term education is required - the American College of Medical Genetics and Genomics and other professional societies have posted numerous guidelines for working with genomic data and understanding it. Additionally, there may be a need for a genetic specialist to join the hospital staff. Considering the workload created by ordering genetic tests, managing patients with genetic conditions, and the integration of this process into the day-to-day workflow, prepare to introduce this new role soon.
The road ahead: challenges to overcome
It hasn’t been long since the start of precision medicine development in 2015 and we already have some results to celebrate. Today, it’s evident to all players that we need to push forward together. Let’s sum up the main challenges the industry keeps working through.
Data sharing and security. Striking a balance between open data and data safety is a difficult task that will lie on the shoulders of primary care gives who need to communicate the change to patients, receive their consent, and then be responsible for securing their data on a day-to-day-basis.
Policy and legislation. Although interoperability final rules will begin to come into effect within months, it will take years for the whole ecosystem to adopt them all and change to a more innovation-driven mindset.
Electronic Health Records. The current design and inconsistencies of EHRs make it difficult for physicians who not only must keep up with the upgraded healthcare system, but also interact with patients. Improving an EHR is a top-priority task for hospitals that want to stay innovative.
Skills and knowledge. Data literacy starts from the moment a patient enters the clinic and follows every step of diagnostics and care. Practitioners need proper infrastructure, must know how to use it, and - preferably - be open to the idea of dramatic change.

Maryna is a passionate writer with a talent for simplifying complex topics for readers of all backgrounds. With 7 years of experience writing about travel technology, she is well-versed in the field. Outside of her professional writing, she enjoys reading, video games, and fashion.
Want to write an article for our blog? Read our requirements and guidelines to become a contributor.

